Abstract
Background: Multiple myeloma (MM) is an incurable plasma cell malignancies despite the advent of numerously new drugs. Survival was poor particularly for high risk patients such as R-ISS stage III. The preliminary data from our center showed that the median PFS after auto-HSCT was 24 months for patients with R-ISS III stage, while 17 months for patients that achieved PR or less after induction. Chimeric antigen receptor (CAR)-transduced T cells is a promising strategy for cancer immunotherapy. Our previous study showed good response for RRMM patients after CD19 and BCMA-specific CART therapy without severe CRS and other deadly side effects. To improve the survival of high risk patients, this study was designed to observe the safety and efficacy of combined infusion of CD19 and BCMA-specific CART cells after autologous transplantation (SZ-MM-CART02 study, NCT 03455972).
Methods:18-65y NDMM in R-ISS stage III, or who only achieved PR or less after 4 cycles of PAD triplet induction were enrolled with serum creatinine (Cr) <2.0 mg/dL, and adequate hepatic, cardiac and pulmonary function. BCMA and CD19 expression on MM cells were analyzed by flow cytometry. Lymphocytes were collected from PBSCs and cultured with an anti-CD3 monoclonal antibody to activate T-cell proliferation. The cells were transduced with recombinant lentiviral verctors which respectively contained the anti-BCMA or anti-CD19 single chain variable fragment (scFv), the cytoplasmic portion of the OX40 and CD28 costimulatory moiety, and the CD3z T-cell activation domain. This is the new third generation CAR technique applied in clinic. BUCY were used as conditioning, followed by infusion of autologous stem cells. Median time to engraftment were 10 days for nutrophils. CART-19 (1×107/kg on d0) and CART-BCMA cells as split-dose (40% on d1 and 60% on d2) were infused derectly on d14 to d20 after autologous transplantation. Levels of CAR-transduced cells are measured by qPCR. The cytokine release syndrome (CRS) was graded according to the UPen cytokine release syndrome grading system. Neurotoxic side effects and other toxicities were assessed according to the CTCAE v 4.03. Plasma levels of IL-2, IL-4, IL-6, IL-10, TNF-alpha, IFN-gamma, and IL-17A proteins were determined with a cytokine kit. Imids alone were given as maintenance therapy. Responses were assessed by IMWG criteria. 10-color flow cytometry was used to monitor MRD regularly after CART treatment. The median of follow-up was 3 (2∼11) months.
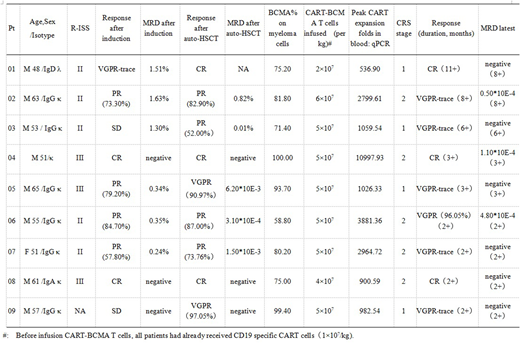
Results: To date, 9 patients have completed the CART cells infusion (cohort 1). All cases expressed BCMA >50% without CD19 expression on MM cells. CRS occurred in 9 patients (100%) grade1 or 2 associated with fever(n=9), fatigue (n=9), elevated IL-6 and CRP (n=9), elevated ALT (n=1, grade 1). Two patients needed to use low-dose vascular active drugs (pts 04 and 07). Other toxicities to date included coagulopathy (n=6, grade 1 for 4 pts and grade 2 for 2 pts), elevated troponin T (n=4, grade 1), and atrial flutter (n=1). There was no serious CRS or neurologic complications occurred in this group of patients.
The ORR was 100% with all patients were monitored for a period of more than 2 months, which may be eventually further improved. There were 2 CR, 1 VGPR, 4 PR, 2 SD after induction; 3 CR, 2 VGPR, 4 PR after APBSCT; 3 CR, 6 VGPR after CART therapy. MRD negativity in BM increased from 37.5% after transplantation to 66.7% after CART therapy latest. Four patients (pts 02, 03, 06 and 07) obtained partial PR after transplantation, and got VGPR after CART cells infusion. We found dramatic in vivo CART expansion that median of peak value of CART copies was 1059.54 folds (ranged from 536.90 to 10997.93 folds) which was 100 folds to that with RRMM patients in our previous study.
Conclusions: Tandom autologous transplantation and combined infusion of CART-19 and CART-BCMA cells could be another choice of consolidation treatment for high risk MM patients. Toxicities to date including CRS and organ function impairment seemed to be mild and reversable. It is worthy of further study to compare DFS, OS between single autologous transplantation and tandom transplantation with CART therapy. Immune environment in high risk patients with multiple myelomaI remodelled by auto-HSCT may contribute to more rapid expansion of CART cells than that in RRMM patients, suggesting that the extent of CART expansion depends more than tumor burden.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal